Impact of brown fat activation during pregnancy on fetal metabolic health
I. Biological Characteristics of Brown Adipose and Metabolic Regulation Mechanisms
Brown Adipose Tissue (BAT) is a unique thermogenic adipose tissue in the human body. Unlike common white adipose (which stores energy), the core function of brown adipose tissue is to break down fat and release heat through mitochondrial uncoupling protein 1 (UCP1).
Structural features: Brown adipocytes are rich in mitochondria (about 40% of the total cell volume) and tiny lipid droplets, and their high-density capillary network ensures efficient oxygen and nutrient supply.
Physiologic Role:
Thermogenesis regulation: In its activated state, brown adipose burns an additional 300-500 kcal per day, which is equivalent to the energy expenditure of jogging for 5 kilometers.
Metabolic protection: high levels of BAT activity are directly associated with lower visceral adiposity, superior insulin sensitivity and cardiovascular health.
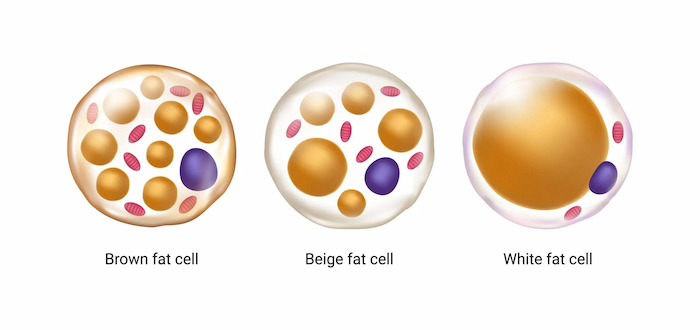
Key Finding:
The percentage of brown fat in the body can reach 5% of body weight in newborns, but only 1-2% remains in adulthood, and the distribution is limited to the clavicle, neck, and around the spine.
Genetic Programming Window Period: animal studies have shown that the embryonic period to 2 years after birth is a critical stage of BAT development, and nutritional and environmental factors at this stage can permanently affect its function.
II. Metabolic Programming Effects of Season of Conception
- Long-term effects of maternal ambient temperature on offspring metabolism
A Japanese study published in Nature Metabolism found that:
Offspring conceived during the cold season (October-April) had significantly higher BAT activity in adulthood than those conceived during the warm season (78.2% vs 66%), and had a 30% increase in metabolic efficiency.
Mechanism: Cold environment induces maternal epigenetic modifications (e.g., DNA methylation) and enhances the expression of fetal BAT-related genes (e.g., PRDM16, UCP1), resulting in a “metabolic memory.
- Transgenerational genetic effects of paternal cold exposure
Mice: Male mice exposed to 4℃ for 14 days prior to mating maintained normal body weight on a high-fat diet, and BAT activity was 3-fold higher than that in the control group.
Sperm epigenetic modification: Cold stimulation promotes BAT production by altering sperm miRNAs (e.g. miR-449c) regulating progeny adipocyte differentiation signaling pathway (WNT/β-catenin).
Clinical implication: appropriate low-temperature exposure (e.g., outdoor activities in winter, avoidance of excessive heating) during pregnancy preparation may optimize offspring metabolic health.
III. Early life intervention: three strategies to compensate for congenital deficiencies
- Nutritional programming during pregnancy
Key nutrients:
Omega-3 fatty acids (deep-sea fish): enhance BAT progenitor cell differentiation and boost UCP1 expression5.
Vitamin A (carrots, spinach): activates BAT-specific gene transcription via retinoic acid receptor (RAR)5.
Resveratrol (grapes, blueberries): promotes “browning” of white fat and increases thermogenesis5.
Dietary contraindications: high-fat diets (>10% saturated fat) can inhibit fetal BAT development, leading to a 40% increased risk of metabolic syndrome in adulthood5.
- Metabolic regulation during breastfeeding
Breast milk composition optimization:
Leptin: regulates the hypothalamic energy balance center and sets the metabolic “set point” for the offspring.
Inositol: improves insulin sensitivity and reduces secretion of inflammatory factor (TNF-α) in adipocytes.
- Environmental and behavioral interventions
Cold acclimatization: short daily cold exposure in infancy (e.g., 15 minutes at 18°C) activates BAT, with effects lasting into adulthood.
Exercise-induced browning: Moderate aerobic exercise (e.g., swimming, yoga for pregnant women) 3 times per week from mid-pregnancy onward promotes fat browning through irisin signaling.
IV. Risky behaviors during pregnancy and metabolic intergenerational harms
- Overnutrition and gene silencing
High-fat diet model: offspring of mice fed a high-fat diet during pregnancy have a 50% increase in methylation levels of thermogenic genes such as Acaa and Cox7a1 in BAT and a 30% decrease in metabolic rate5.
Clinical recommendations: weight gain during pregnancy should be controlled within the IOM criteria (11.5-16 kg for normal BMI) and avoiding calorie intake of more than 3000 kcal in a single day.
- Transgenerational programming of stress hormones
Glucocorticoid exposure: Dexamethasone injection (simulated stress) in pregnant rats resulted in a 40% reduction in BAT mitochondrial density and reduced thermogenesis in the offspring.
Interventions:
Stress management: 20 minutes of daily meditation reduced cortisol levels by 25%5.
Drug replacement: discontinue glucocorticoids before pregnancy and switch to hydroxychloroquine (immunomodulator) to control inflammation.
V. Future direction: precision medicine and gene editing technology
- Epigenetic marker detection
Placental DNA methylation analysis: Predict the risk of obesity in offspring by detecting the methylation status of BAT-related genes (e.g. UCP1, DIO2).
Personalized Nutritional Program: Customized Omega-3 and antioxidant supplementation program based on maternal metabolomic data.
- CRISPR-Cas9 gene editing
Animal experiment breakthrough: Successfully transform white fat into functional BAT by targeting and editing the PRDM16 gene in fetal BAT progenitor cells.
Ethics and Challenges: Need to balance the technical potential and unknown risks, currently limited to the scientific research stage.
VI. Conclusion: A scientific pathway from innate programming to lifelong health
Brown fat activation is not only the “starting line” of fetal metabolic health, but also the window of life for intervention. By optimizing the conception environment, precise nutritional intervention and stress management, a strong metabolic defense system can be built for the offspring even if the ideal conception season is missed. With the advancement of epigenetics and genetic technology, every family will have a customized plan in the future, truly realizing “health from the very beginning of life”.
相关推荐
- Seven Differences Between IVF and Natural Pregnancy
- 5 Life and Death Decisions to Ask About Embryo Talk
- Preventing and resolving Down’s syndrome: A global breakthrough in IVF technology and hope for the future
- The Ideal Childbearing Age for Surrogacy
- The Preparedness Revolution: The Lancet’s Disruptive Study Reveals the Truth About Health That Affects Three Generations of Children and Grandchildren
Search within the site
Surrogacy News
Hot Tags.
Kyrgyzstan Surrogacy Agency,Global IVF Hospitals,International Surrogate Mother Recruitment